NAD and Mitochondria: The Key to Unlocking Aging and Neurodegenerative Diseases
An international research team led by Professor Mathias Ziegler from the Department of Biomedicine at the University of Bergen (UiB) has made a significant discovery about Nicotinamide Adenine Dinucleotide (NAD). Their findings shed light on how NAD levels influence aging, cellular health, and the development of neurodegenerative diseases.
NAD Powers Cells and Repairs Damage
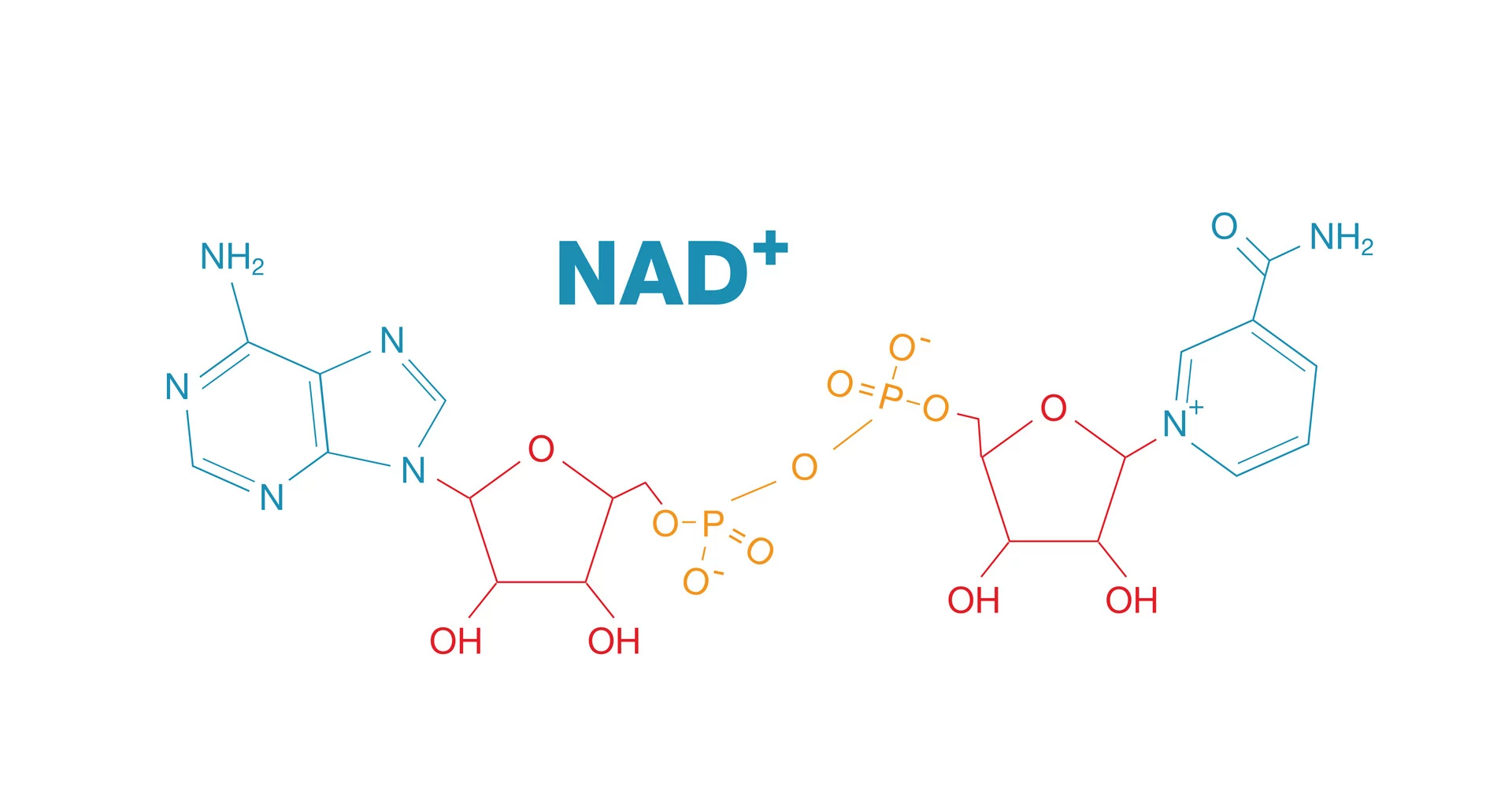
NAD plays a vital role in every cell. It acts like a rechargeable battery, capturing energy from food and distributing it to fuel essential functions. Most of this process happens in mitochondria, often called the cell’s powerhouse. Beyond energy transfer, NAD regulates gene expression and repairs DNA, protecting cells from damage.
As we age, DNA accumulates more damage, increasing the demand for NAD. Ziegler explains that this heightened activity depletes NAD levels over time. When cells can’t meet this demand, especially when mitochondria lose their ability to replenish NAD, energy shortages lead to dysfunction and disease.
Mitochondria as an NAD Reservoir
To study how cells handle declining NAD levels, Ziegler’s team developed innovative models and applied advanced techniques like CRISPR-Cas9 and high-resolution mass spectrometry. They discovered that mitochondria act as an NAD reservoir, storing energy during normal function and releasing it when cells need extra support.
“Our research shows that mitochondria actively refill their NAD reserves and keep the cell supplied,” says PhD student Lena Høyland, the study’s lead author. However, when cells overuse this reserve for extended periods, mitochondria can no longer meet the demand.
“Prolonged stress on mitochondria drains their NAD supply,” Høyland adds. “This lack of energy disrupts vital processes and can lead to severe consequences for cellular health.”
New Hope for Aging and Disease

Ziegler’s research connects mitochondrial dysfunction and NAD depletion to aging and diseases like dementia and neurodegeneration. His team believes that excessive NAD consumption within mitochondria may accelerate these conditions.
Encouragingly, recent clinical trials have shown that NAD supplementation can restore cellular energy and slow the progression of age-related diseases.
“We’re excited to uncover this mechanism and its impact on disease,” says Høyland. Ziegler concludes, “Our findings highlight how basic research can reveal targets to combat aging and develop effective treatments.”
Published in Nature Metabolism, this study offers fresh insights into how cells age and paves the way for innovative therapies to tackle aging-related diseases.